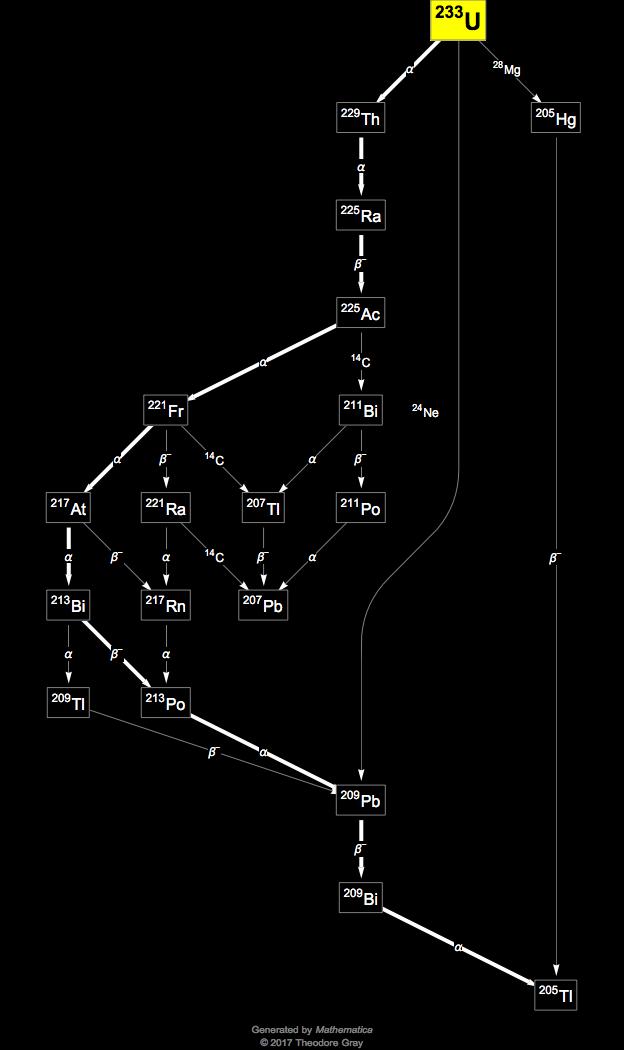
What Is The Half Life Of Uranium . Uranium is a radioactive element and conteneously disintegrate into smaller element, that time in which 1g of uranium becomes half g is known as half life period of uranium. In other words their nuclei spontaneously disintegrate or decay.
Radioiodine. Causes, symptoms, treatment Radioiodine from dxline.info
Its specific activity is very high ~22 ci/g and its. Why does uranium have a long half life? Uranium 238 occasionally decays by spontaneous fission with probability of 0.000055%.
Radioiodine. Causes, symptoms, treatment Radioiodine
238 u cannot support a chain. Why does uranium have a long half life? Its specific activity is very high ~22 ci/g and its. Though both isotopes were at the time of earth formation equally abundant, natural uranium today consists today of 99.3%.
Source: voyager.jpl.nasa.gov
Check Details
Uranium 235 occasionally decays by spontaneous fission with very low probability of 0.0000000072%. Uranium is weakly radioactive because all isotopes of uranium are unstable; Most uranium is u238 which is more stable — 4.4 billion years half life. 238 u cannot support a chain. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons.
Source: dxline.info
Check Details
Uranium has three main radioactive elements; So if there 1kg of u238 6.3 billion years ago, about one third of the u238 atoms would still not have decayed. The earliest study he cites is nier (1939) (a. Uranium 232 very rarely decays by spontaneous fission. Why does uranium have a long half life?
Source: voyager.jpl.nasa.gov
Check Details
Why does uranium have a long half life? A critical review, by norman holden, which reviews various early studies of each of the common isotopes of uranium (232 u, 233 u, 234 u, 235 u, 236 u, and 238 u). Unlike thorium, some of uranium isotopes undergo fission. Its specific activity is very high ~22 ci/g and its. The earliest.
Source: www.sciencephoto.com
Check Details
Most uranium is u238 which is more stable — 4.4 billion years half life. Its specific activity is very high ~22 ci/g and its. A critical review, by norman holden, which reviews various early studies of each of the common isotopes of uranium (232 u, 233 u, 234 u, 235 u, 236 u, and 238 u). Unlike thorium, some of.
Source: periodictable.com
Check Details
Most of them exist for no more than a few seconds, and some are very much smaller. Why does uranium have a long half life? Uranium 238 occasionally decays by spontaneous fission with probability of 0.000055%. Though both isotopes were at the time of earth formation equally abundant, natural uranium today consists today of 99.3%. This means that in 4.46.
Source: www.mentalfloss.com
Check Details
Most uranium is u238 which is more stable — 4.4 billion years half life. Uranium 238 occasionally decays by spontaneous fission with probability of 0.000055%. Uranium 235 occasionally decays by spontaneous fission with very low probability of 0.0000000072%. Uranium is a radioactive element and conteneously disintegrate into smaller element, that time in which 1g of uranium becomes half g is.
Source: images-of-elements.com
Check Details
A critical review, by norman holden, which reviews various early studies of each of the common isotopes of uranium (232 u, 233 u, 234 u, 235 u, 236 u, and 238 u). 232 u very rarely decays by spontaneous fission. So if there 1kg of u238 6.3 billion years ago, about one third of the u238 atoms would still not.
Source: www.lanl.gov
Check Details
This means that in 4.46 billion years, only half of the uranium would have decayed. In other words their nuclei spontaneously disintegrate or decay. Uranium is a radioactive element and conteneously disintegrate into smaller element, that time in which 1g of uranium becomes half g is known as half life period of uranium. Uranium 238 occasionally decays by spontaneous fission.